CAR T-cell Therapy: A Breakthrough in Personalized Medicine for Cancer
Cancer remains a prevalent concern in India, with one in nine individuals likely to face this problems in their lifetimes. In the ever-evolving landscape of cancer treatment, CAR T-cell therapy emerges as a groundbreaking and personalized approach, leveraging the body's immune system to combat the disease.
What is CAR T-Cell Therapy And What Is New In It?
CAR T-cell therapy stands out in cancer treatment, departing from traditional methods like surgery, chemotherapy, and radiation therapy. The 2000s witnessed a paradigm shift with the introduction of targeted therapy and immunotherapy. CAR T-cell therapy takes a distinctive approach by harnessing immune T-cells and modifying them to enhance their ability to detect and combat various threats, including cancer cells.
How Does CAR T-Cell Therapy Work?
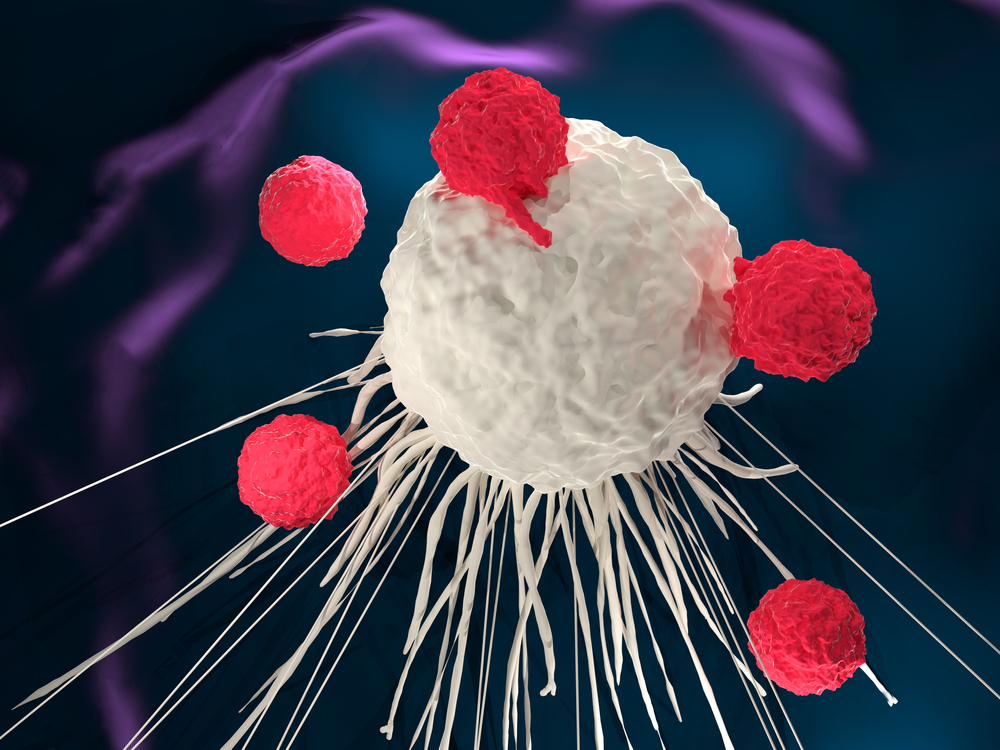
The therapy involves engineering T-cells with a Chimeric Antigen Receptor (CAR) on their surface. These engineered T-cells can be sourced from the patient's blood (Autologous) or a donor (Allogenic). The CAR is designed to recognize and bind with specific proteins found exclusively on the surface of cancer cells.
This modification turns T-cells into powerful agents with dual functionality, acting like a twin drug. The engineered T-cells are then multiplied in a lab, and these expanded cells are introduced into the patient's body through intravenous infusion. Once inside the patient, these CAR T-cells continue multiplying, guided by their engineered receptors. This proliferation allows them to identify and eliminate any cancer cells expressing the specific protein targeted by the CAR.
These cells act as therapeutic agents within the patient's system, traveling throughout the body, recognizing and engaging with cells expressing the specific protein associated with cancer. Once engaged, the CAR on the T-cell activates and eliminates the targeted cancer cells. In summary, T-cells are modified in a lab to recognize cancer cells, and these modified cells are then infused into the patient's body. They continue to grow inside the patient, locating and precisely destroying cancer cells.
Who Are the Primary Candidates For CAR T-Cell Therapy?
A significant portion of CAR T-cell therapy research has concentrated on leukaemias and lymphomas, typically involving patients who have faced treatment failures, including bone marrow or stem cell transplants. Eligibility for CAR T-cell therapy extends to patients experiencing the recurrence of leukaemia or lymphoma.
More recently, CAR T-cell therapy has gained approval for use in multiple myeloma as well. Examination of these tumors reveals their association with blood or blood-like structures in the body, such as lymph nodes, primarily connected to the immune system within the lymph nodes. While research in the context of solid tumors has been conducted, the outcomes have been less successful for several reasons.
Solid tumors generally lack a singular protein or antigen effectively targetable by CAR T-cells, and the physical structure of solid tumors poses challenges in delivering CAR T-cells to their core. Consequently, CAR T-cell therapy has not demonstrated comparable success for solid tumors present in organs like the lungs or breast.
However, CAR T-cell therapy has achieved significant success in treating relapsed and refractory acute lymphoblastic leukemia, B-cell non-Hodgkin’s lymphomas, certain lymphomas like mantle cell lymphomas, and multiple myeloma. These are the approved indications to date, but ongoing research is expanding the spectrum of tumors under investigation. As scientific understanding progresses, CAR T-cell therapy is anticipated to be applied to a broader range of cancer types.
What Are The Side Effects of CAR T-Cell Therapy?
Infusing CAR T-cells into the body can trigger reactions and side effects, with the most frequent and severe being cytokine release syndrome (CRS). Although these side effects are commonly associated with the engagement of CAR T-cells with target cells, especially in combating cancer, they are generally accepted in the majority of cases. The side effects linked to CRS result from specific chemicals released by CAR T-cells during their interaction with cancer cells.
Despite the associated challenges, these side effects are generally accepted as indicators of the therapy's activation against cancer cells. Effective management strategies, including specific medications and close monitoring during hospitalization, help mitigate these side effects.
Are These Side Effects Manageable?
Doctors employ various strategies to manage these side effects, and patients are typically kept in the hospital for monitoring and treatment. Effective methods to mitigate these side effects include the use of specific medications during hospitalization.
In the case of cytokine release syndrome (CRS), which involves inflammation in internal organs, drugs are administered to counteract this inflammation, particularly in the brain, lungs, and other parts of the body. These drugs may include steroids and certain monoclonal antibodies designed specifically to address CRS.
Notable Success And BreakThroughs Observes With CAR T-Cell Therapy
CAR T-cell therapy stands as an established and remarkably effective treatment for patients with relapsed acute lymphoblastic leukemia, as well as certain relapsed lymphomas and myelomas.
These individuals, having exhausted various treatment options, currently have limited therapeutic alternatives available to them for either management or a potential cure. CAR T-cells represent a remarkable success story in the realm of medical science, marking the forefront of innovative advancements.
Future of CAR T-Cell Therapy And Its Impact On Cancer Treatment
Research in CAR T-cell therapy is an ongoing and rapidly evolving field, with numerous clinical trials underway, driven in part by the identification of additional targets on tumor cells. This is promising for the development of future CAR T-cell therapies.
So far, two primary antigens, CD19 for leukemia and lymphoma and BCMA for myeloma, have been utilized in currently approved CAR T-cell therapies, totaling around half a dozen. As new targets, especially in tumor types like breast, kidney, and brain cancer, are identified, further advancements are anticipated.
These developments are significant successes in the realm of medical science. It's noteworthy that CAR T-cell therapies face challenges in treating solid tumors due to the unique microenvironment in which these tumors exist.
Scientists are actively working on designing solutions to enhance the effectiveness of CAR T-cell therapies in solid tumors. As our understanding continues to expand, the growth of CAR T-cell therapies is expected, potentially encompassing a broader range of tumor types in the future.
Conclusion
In conclusion, CAR T-cell therapy is a revolutionary treatment option to fight cancer. It shows all the potential signs of success and is paving its way to be a leading treatment for cancer worldwide. The revolutionizing success of this personalized medicine for cancer has shown that no disease should ever hold us back from living our fullest life.
This blog has been converted from the PR Article - What’s CAR T-cell therapy, a cutting edge breakthrough in cancer treatment